|
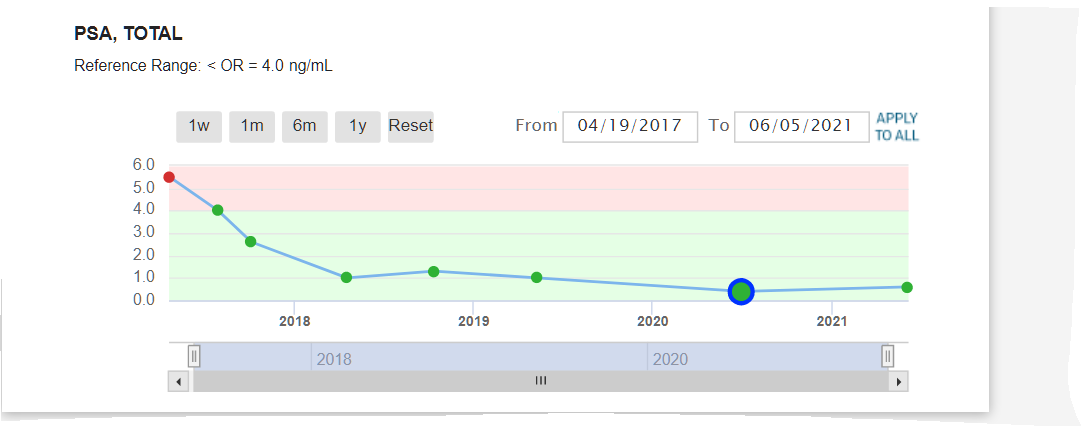
As you can see, results are great from 2017 to 2023 staying around .01 ~~ They will only do a PSA test every year now so it comes due Oct 2024 where I know it will be just fine. No symptoms at all to report. Life is Good.
0 Comments
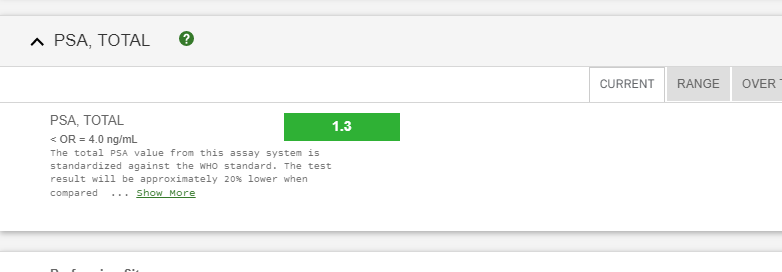
Sorry no updates for the last several years.. Results 2024 ~~ Its Still ALL GOOD Results same as 2020 PSA 1 still not any side effects... Life is Good... Debbie and I still walk at least 2 times week on Siesta Key Beach (Paradise - #1 beach in the US) - walk 4 miles - which is something we started the 6 weeks I had treatments. It relaxes us, we stay fit and just love being together. We continue to eat organic and take supplements that keep us free of cancer and will not ever change that. We know what you eat and drink makes a huge difference. She posts the diet and supplements and Health Care Info on her website she started 18 yrs ago.
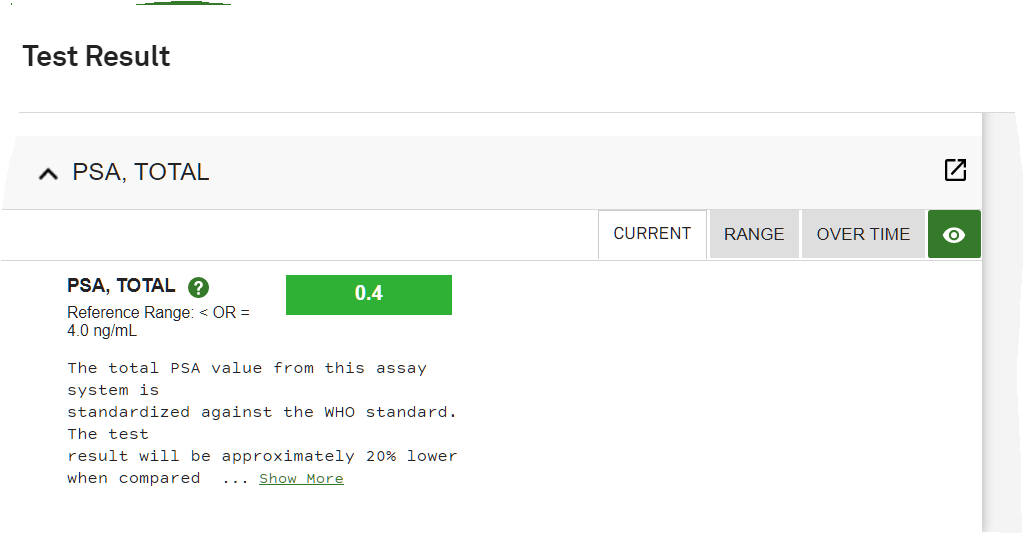
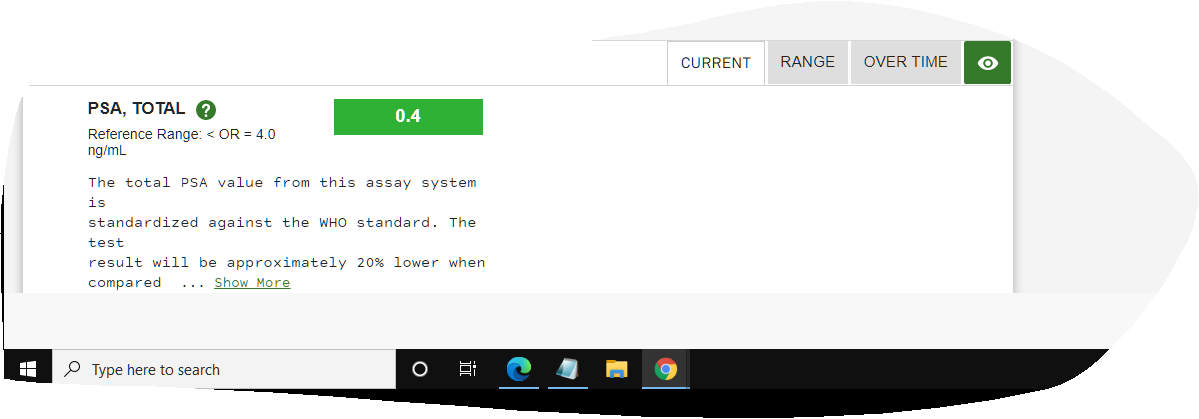
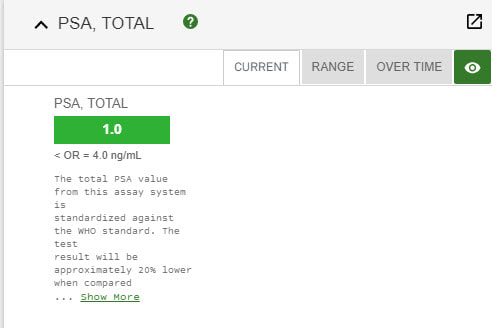
The most important part of this post is to let everyone know that there have been NO side efects whatsoever during (very minor when I started it) or since my Proton Therapy was completed. What an awesome thing. Thanks to the changes in my diet, Proton Therapy at UF Proton Therapy Institute with incredible doctors and staff, plus my incredible wife pushing me and at my side, I am cancer free and happier and healthier than I have ever been. Still playing softball and won my last 4 tourneys 4-0. Life is Good. Results 2021 ~~ Its Still ALL GOOD Results same as 2020 Still not any side effects... Results below. Life is Good... Debbie and I still walk at least 2 times week on Siesta Key Beach (Paradise - #1 beach in the US) - walk 4 miles - which is something we started the 6 weeks I had treatments. It relaxes us, we stay fit and just love being together. To be honest, we both said we had even forgotten I had Prostate Cancer. I know Debbie said she had not thought about it since we got back because she knew it was gone. She never doubted it would not be. I have to agree with her now. Still an amazing feeling. (Unfortunately, she went thru cancer 15 years ago and had treatments for several years, so it was much different for her - she is cancer free and has been for many years now.)
Thank to Proton Bob for this New Information - check out his website - link at bottom of article Transperineal vs. Transrectal Prostate Biopsy: Significant New Developments
Last month’s story about BOB member “Lee” shined a light on a serious problem associated with the standard transrectal biopsy procedure routinely performed around the world every day. This prompted several emails from members who had similar experiences. One informed us of a new and relatively unknown technology that virtually eliminates the sepsis infection problem. This piqued our interest, so we followed up on the lead. And we’re glad we did. Our research revealed that about two million Americn men undergo conventional transrectal biopsies each year and up to 2,000 of them die from the procedure, mostly from sepsis infections, not to mention bleeding complications associated with the procedure. Hospitalization for sepsis costs between $9,000 and $19,000 per patient according to Richard Szabo, MD at the University of California, Irvine. The Journal of Endourology reports “many centers around the world have adopted fTP-Bx (“free-hand” transperineal prostate biopsy) because it virtually eliminates sepsis, may improve detection rates of clinically significant prostate caner, and can be easily integrated into a normal clinic workflow using only local anesthesia.” The journal also reports that if all urologists in the U.S. abandoned transrectal biopsies for transperineal biopsies, “the potential savings in healthcare costs of complications would be significant.” The PrecisionPoint™ Transperineal Biopsy Deb and Bob met via Zoom with Dr. Matthew Allaway, a urologist and inventor of the PrecisionPoint™ transperineal biopsy device and procedure, along with Ken Knudson, CEO of his new company, Perineologic, and Evan Brasington, CCO. Following is what we learned from the meeting. Over the past 18 years, Dr. Allaway has been caring for men, women, and children with a myriad of urologic conditions in the small town of Cumberland, MD. “As a urologist,” he said, “I treat a wide spectrum of conditions that span from urinary infections and kidney stones, to cancers of the kidney, bladder, and prostate using modern advancements to improve outcomes. I’m committed to contemporary screening methods for prostate cancer and the potential benefits of early diagnosis and patient-directed treatments.” The conflict he struggled with was accepting the known complications and limitations with the most important first step in the treatment of prostate cancer, the prostate biopsy. Once prostate cancer is suspected, either through an increase in PSA and/or confirmatory prostate MRI, a biopsy of the prostate is required to establish the diagnosis of prostate cancer. Almost two million prostate biopsies are performed in the U.S. each year. Dr. Allaway’s research and efforts have been devoted to developing a better, safer biopsy for prostate cancer. The Standard Biopsy and Associated Risks The current standard of care for a prostate biopsy is a transrectal ultrasound-guided (TRUS) biopsy. It requires the urologist to take samples of the prostate by passing a needle through the rectum of the patient in order to access the prostate. This procedure runs the risk of infecting the patient with fecal matter introduced into the prostate with each pass of the biopsy needle. Generally, patients are given antibiotics to reduce this risk, but due to societal overuse of antibiotics, up to 25 percent of bacteria are resistant to the antibiotics given. The trans-rectal biopsy typically requires 12 needle sticks through the rectum to access the prostate for biopsy samples. Dr. Allaway said, approximately 1-6 percent of patients worldwide develop a serious sepsis infection requiring hospitalization. Some publications place the sepsis infection rates higher. One article from the Sperling Prostate Center reported TRUS biopsy caused infections as high as 7.2 percent. Another study published in the U.S. National Library of Medicine reported urosepsis following TRUS prostate biopsy to be even higher. “Sepsis is a serious complication of infection and between 12-25 percent of cases of sepsis are fatal,” said Dr. Allaway. “Other serious complications of the transrectal biopsy are injury to blood vessels of the rectal wall resulting in rectal bleeding. Less serious risks include temporary inability to urinate, pain, and erectile dysfunction. I decided these risks were unacceptable for my patients,” he told us. His second concern with the transrectal approach was the limitations in sampling the entire prostate. “Up to 30 percent of cancer is either not identified or mis-classified,” said Dr. Allaway, “Missing the cancer often leads to repeated biopsies in order to capture the cancer, exposing the patient once again to the complications. Misclassification could result in the patient being treated as having low-risk disease, when in fact, they harbor higher-risk disease that will need active treatment.” A Better, Safer Biopsy To address these risks, Dr. Allaway developed a device and surgical method called the PrecisionPoint™ Transperineal Access System (PPTAS) which allows the urologist to perform the prostate biopsy in a revolutionary way that eliminates the complications and risks associated with the transrectal method of biopsy. “This system uses a safer route for taking samples of the prostate by passing an access needle through the perineum or taint. From there, the prostate is sampled thoroughly with the patient experiencing only two needle sticks and little or no discomfort,” said Dr. Allaway. “The risk of infection using this approach is reduced to nearly zero and the complications of rectal injury are eliminated.” While his major focus was to eliminate the risk of infection, another equally important benefit of this approach presented itself. That benefit included better access to difficult-to-reach parts of the prostate gland during the biopsy, resulting in nearly 30 percent better cancer detection rates. “We’ve treated more than 3,000 patients in the Cumberland area with this method and have had patients visit us from around the world to have their biopsies in our clinic.” The New Standard of Care? The revolutionary PrecisionPoint method is quickly being adopted around the world. Twelve of the 15 U.S. News and World Report’s top-rated urology centers are using the PrecisionPoint Transperineal Access System. Additionally, 12 countries outside the U.S. are using the device and method. Many of the countries have made the commitment to eliminate the transrectal biopsy and use the PrecisionPoint biopsy as the standard of care. “We are working to make the PrecisionPoint biopsy the standard of care throughout the U.S. so that all men can have the benefits of a better, safer biopsy and relegate the trans-rectal biopsy to the medical archives,” Dr. Allaway said. “We’re expecting more than 40,000 prostate biopsies in the U.S. will use PPTAS technology in 2021.” Educating Urologists and Patients Men and their family members are becoming aware of the options and are requesting the transperineal approach. Unfortunately, over 90 percent of biopsies in the U.S. are still performed using the transrectal pathway. Many urologists simply are not aware of this transperineal option and therefore patients are not informed of their options. Outside the U.S., the transrectal method is being actively phased out and the European Association of Urologists have changed their guidelines, recommending that the transperineal biopsy be offered. “We intend to continue our mission by training urologists on the PPTAS, educating patients and working with insurance carriers, Medicare, and the American Urologic Association to offer the very best and safest biopsy.” Transperineal biopsies are not new. They have been done before, but the procedure involved the use of a stepper device to penetrate the perineum 18 to 24 times and it required general anesthesia. And while this procedure resulted in far fewer infections, it was expensive, time consuming and therefore never gained wide popularity and use. The PrecisionPoint technique uses a new device that is relatively simple to use, requires only two punctures of the perineum for multiple biopsy samples and can be done under local anesthesia. The procedure is done in roughly the same amount of time as the TRUS procedure and costs about the same with the addition of a $200 disposable PPTAS device. One urologist we spoke with about the PrecisionPoint transperineal biopsy called it a “game changer.” Learn more. https://perineologic.com/ https://protonbob.com/2021-05-protonbobtales Thanks for sharing this Exciting News and breakthrough technology Proton Bob ~~ Check out his website and become a member - his link is at the bottom of this article. Theranostics ─ A Bold New Tool for Fighting Cancer
As mentioned in our opening memo, theranostics is a breakthrough technology you’ll be hearing a lot about in the future. Loma Linda University Health (LLUH) is collaborating with BAMF Health on this venture. Both institutions are in the process of establishing theranositc centers at their respective headquarters. Both LLUH and BAMF are already collaborating on developing new theranostic agents. The following article was written for the BOB Tales by Mark Reeves, MD, PhD, Cancer Center Director at LLUH. Dr. Reeves is in the process of establishing a Comprehensive Cancer Center at Loma Linda, designated by the National Cancer Institute. One of their initiatives is to pursue and develop this new technology. The article is somewhat technical for many of our members, but the importance and significance of this new technology is clearly evident. We encourage you to carefully read this important article and consider the implications of using this breakthrough technology for treating advanced, metastatic prostate cancer as well as a whole range of other cancers previously untreatable with today’s technologies. This new technology is totally consistent with the proton therapy concept of destroying disease while minimizing damage to surrounding healthy tissue. And you’ll see in this article how theranostics and proton therapy can, and will be, synergistic technologies. This is truly exciting news. Theranostics By Mark Reeves, MD, PhD, Cancer Center Director, LLUH Theranostics is an emerging field that combines molecular therapies (therapy) and paired molecular targeted imaging (diagnostics). In oncology, a molecule that binds to a tumor is first used to image the cancer, and then that same molecule is used to destroy the cancer. This approach is being used to transform many fields in medicine, especially neurosciences and oncology. It has the promise of moving oncology from “trial-and-error medicine” to “precision medicine.” An Example Theranostics is already transforming the diagnosis and treatment of rare, difficult-to-treat neuroendocrine tumors. We have known for a long time that they universally bind to a molecule called “somatostatin.” Based on this, a version of somatostatin called “DOTATATE” is connected to diagnostic radioisotopes (68Ga) that can be detected using positron emission tomography (PET/CT). Thus, 68Ga-DOTATATE is injected into a patient with a neuroendocrine tumor, and a very specific PET/CT is obtained that finds both the primary tumor and sites of spread that can’t be seen on typical scans. Then, after all the sites of tumor have been identified, the same molecule (DOTATATE) is hooked to a therapeutic isotope (177Lu) that creates lethal radiation in the local area where the DOTATATE binds. 177Lu-DOTATATE is infused into the patient; it seeks out the sites of cancer; and the 177Lu destroys it with localized radiation. It can be thought of as a system that seeks (68Ga-DOTATATE) and precisely destroys (177Lu-DOTATATE) the cancer, leaving other tissue alone. Scratching the Surface This is good, but neuroendocrine tumors are rare. Can theranostics be used for more common cancers such as prostate cancer? Yes. The most well-known example of this is prostate-specific membrane antigen (PSMA). PSMA is a glycoprotein that is highly expressed in prostate cancers. More important, it is more highly expressed in high-risk and metastatic prostate cancer. PSMA is being developed as a theranostic agent to detect and treat prostate cancer. The data supporting 68Ga-PSMA-11 (a ligand that binds to PSMA) PET/CT scanning for prostate cancer are compelling. PSMA PET/CT is far more sensitive than bone scanning or fluciclovine PET/CT, and in one study changed treatment decisions in 62 percent of patients with biochemically recurrent prostate cancer. PSMA is also being developed as a paired therapy (eg: 177Lu-PSMA-11) for prostate cancer. Early data are showing significant responses to PSMA therapy even in heavily pre-treated patients with widely metastatic disease. While not available outside of a clinical trial in the U.S., it is available in Europe. Development of New Compounds Because not every prostate cancer binds PSMA, the development of new compounds that bind to prostate and other cancers is critical. In the new Molecular Imaging and Therapeutics Research Program (MITRP) in the LLU Cancer Center (directed by Frankis Almaguel, MD, PhD), we have used boron chemistry to synthesize and develop molecules that bind to a number of prostate and other cancer biomarkers (eg: enolase, FAPI, MetAP2, etc). These molecules are then developed into theranostic pairs to image and treat cancer. A New Theranostics Center To leverage this theranostic approach to precision cancer therapy, Loma Linda University Cancer Center is developing a Theranostics Center as part of the MITRP in collaboration with BAMF Health. “We are excited to partner with LLUCC to bring this futuristic but real technology to the patients in a high speed manner. This partnership will make a significant impact to the future of cancer care with a strong research capability in proton therapy, health disparity, and other cancer related fields within LLU community,” said Dr. Anthony Chang, founder and CEO of BAMF Health. The Theranostics Center is being developed in three phases: Phase 1, which went live March 1, is a consultation clinic that makes theranostic consultations available to patients under the direction of Dr. Almaguel. Phase 2, which we anticipate being active in 2022, will create a dedicated theranostics clinic, which will contain both dedicated molecular imaging facilities and a precision therapy and infusion clinic. It will allow us to provide both standard and experimental theranostic imaging and treatment. Phase 3, which is planned to be active in 2-3 years, will leverage our existing cyclotron capabilities to create a full theranostics program. This will provide expanded molecular imaging and precision therapy clinics, full synthetic radiopharmacy capabilities, synthesis and translation of novel molecules into early-phase clinical theranostics trials, and the utilization of artificial intelligence to provide more efficient and precise therapies. Particularly exciting about this program is the full translational ability to conceptualize and synthesize compounds in the chemistry lab, develop them in sophisticated animal models, and bring them to early phase human use in a short period. Theranostics and Proton Therapy Adding further to the excitement about theranostics is the obvious connection to proton therapy and other particle therapies such as boron neutron-capture therapy (BNCT). We have already started integrating PSMA theranostics into proton therapy planning, and will combine these modalities in future clinical trials. Imagine for a moment, patients newly diagnosed with prostate cancer receiving theranostic molecular imaging with PSMA or other newer compounds and using these results to decide on the best approach to treatment. For instance, if the theranostic scan showed disease only in the prostate, then proton therapy might be chosen as the best treatment approach. Better yet, if the scan showed that only a specific part of the prostate had disease, then only that part of the prostate might be treated with proton therapy, potentially further lowering side effects. If the scan showed minimal distant metastatic disease and significant disease in the prostate, then the best approach might be up-front therapy with the theranostic agent, which would seek out and destroy the metastatic sites of disease. Proton radiation might then be used to treat any residual disease in the prostate. All these approaches obviously need to be developed and tested in clinical trials, and there are many other potential synergies between theranostics and particle therapy. While this will require time and investment of resources, we believe that the co-location of theranostics and proton centers at LLUH make it the obvious place to develop and deliver these precision cancer treatment approaches. ► BOB Comment: We reached out to Dr. Reeves and asked for more details about the timing of implementation of the theranostics program at Loma Linda that may benefit BOB members with recurrent cancer. He said, “We hope to have our theranostics center up and running and able to provide this therapy in 2022. Patients can be seen before then, however, to determine if they’re candidates for PSMA therapy.” https://protonbob.com/2021-05-protonbobtales Science Resources https://pubmed.ncbi.nlm.nih.gov/23286214/ Results 2020 ~~ Its Still ALL GOOD
|
John MilesOur Journey thru Proton Therapy Archives
May 2024
Categories
All
|













 RSS Feed
RSS Feed